Thesis vs. Non-thesis: What’s the Difference?
Thesis vs. Non-thesis: What’s the Difference?

Thesis vs. Non-thesis: What’s the Difference?
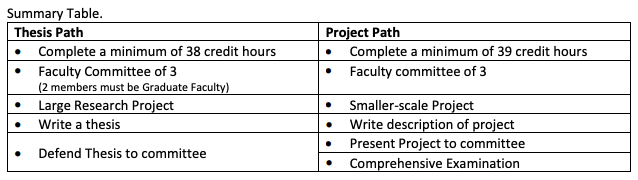
What is the difference between the Regulatory Affairs Thesis and the Regulatory Affairs Project (non-thesis)? Essentially, the thesis option is characterized as the more traditional research option that typically focuses on choosing an original topic, conducting an extensive literature review to delimit the scope of the topic, and to develop research questions that the student seeks to answer. The thesis option requires the researcher to carefully focus on the methodology to be utilized which includes identification of how data will be collected to help answer the research questions or hypotheses and to develop a detailed data analysis plan. The thesis route may take several approaches including the classic experimental design, social research, and policy analysis to mention only a few. Thesis option students will conduct the approved research, write the thesis which summarizes all aspects of the endeavor and submit it to his/her major professor and thesis committee for review and approval. Once the thesis is presented to the student’s committee and successfully defended, the publication will be submitted to the UGA library for archiving. The thesis option is most appropriate for students who desire to develop additional research skills and who plan on pursuing a Ph.D. or seeking a career in research.
The project option oftentimes involves the researcher seeking to solve a problem or issue of significance in regulatory affairs or quality assurance. The methodology employed will vary depending on the scope of the problem chosen but will include an analysis and evaluation of the process involved. The topic chosen may be less novel or original as compared to an identified need. Generating the objectives of the project, defining the intervention approach, data collection and analysis are equally important to the project option. In addition, a comprehensive examination covering the subject matter covered during the degree program is required. Regardless, all RA Master’s students must conduct some sort of research or project to fulfill the degree requirements. All theses and projects must be preapproved by the student’s major professor and faculty committee.
or quality assurance. The methodology employed will vary depending on the scope of the problem chosen but will include an analysis and evaluation of the process involved. The topic chosen may be less novel or original as compared to an identified need. Generating the objectives of the project, defining the intervention approach, data collection and analysis are equally important to the project option. In addition, a comprehensive examination covering the subject matter covered during the degree program is required. Regardless, all RA Master’s students must conduct some sort of research or project to fulfill the degree requirements. All theses and projects must be preapproved by the student’s major professor and faculty committee.
The Project (or non-thesis) option is designed to be more flexible and is tailored for students who don’t necessarily desire more extensive research training. Project students will take an additional credit hour of an elective, and will take a comprehensive examination. Project students must write a project summary document describing their project and present their findings to a faculty committee. This document is typically less involved than the thesis.
NOTE: All Master’s students must complete either a thesis or a Regulatory Affairs Project, which means that each student must have a faculty member to advise on the project. Students are not assigned to a faculty member; instead, the student must take the initiative to contact a faculty member working in their area of project interest. For the Project student, the faculty committee is charged with creating questions for the comprehensive examination in addition to providing guidance throughout the academic endeavor.
Download the .pdf of this document.
Find more information on a graduate degree or graduate certificate in Regulatory Affairs:
Graduate Certificate in International Biomedical Regulatory Sciences
Graduate Certificate in Clinical Trials Design and Management
Master of Science (M.S.) in International Biomedical Regulatory Sciences
